Technical description of the BE-Fuelsaver®:
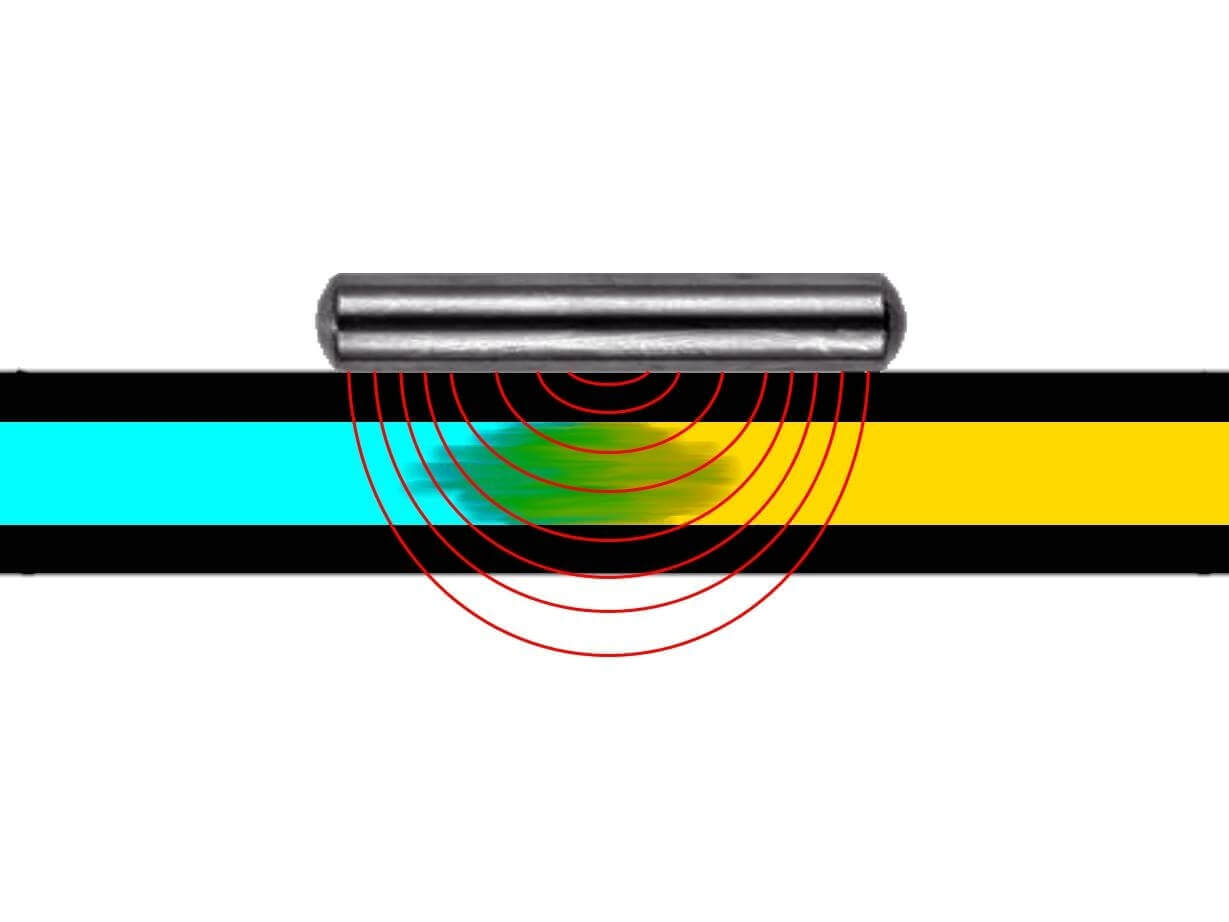
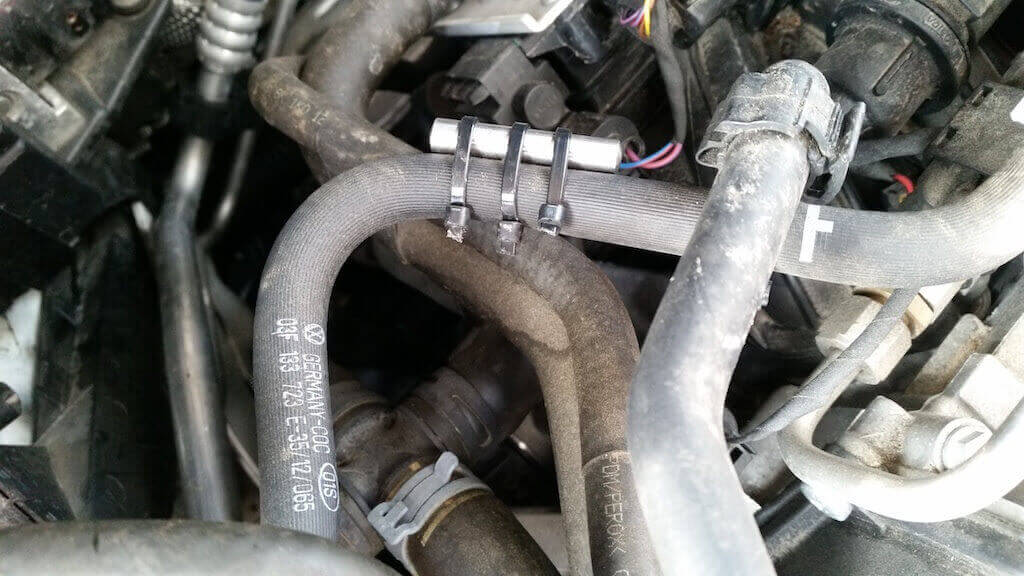
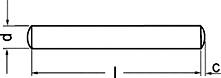
The BE-Fuelsaver® is a metal stick, only a couple of centimetres long, made out of stainless steel (depending on the type, it is 6 to 10 millimeter thick and 26 to 50 millimeter long). It’s placed in a car’s tank or, if need be, the fuel line and reduces the fuel consumption by between 6 and over 20% while also lowering the pollution and improving the engine power.
Detailed description of BE-Fuelsaver®:
Using a special vibration method the BE-Fuelsaver® allows the electrons of the C-C (in black) and C-H connections to go to an energetically higher level. This prepares the respective fuel to be cracked into low-molecular ionised gas in the combustion chamber. A partial plasma is created.
Plasma describes a special state of matter in which the atoms exist in an ionised form, so that when these atoms move they form electrical currents and electro-magnetic fields. In this state the chemical-physical properties are largely changed. Normally these Ion-Electron-Gas-Mixtures only exist in very high temperatures. But through the BE-Fuelsaver® the plasma process starts building much earlier. The exact nature of processes is not yet fully researched, but the clearly measured reduction of toxic emissions like CO, HC, NOx as well as soot-particles shows a change in the combustion process through the partial building of plasma.
In practical use the BE-Fuelsaver® demonstrates the reduction of peak moments through a more homogenous combustion and pressure distribution in the combustion chamber. This can also be seen in the smoother running of the engine.
The components of a typical, four-tact, DOHC piston motor.
Petrol and Diesel are long chain hydrocarbons that contain not only C-H but also C-C bonds. No Oxygen can attach itself to the C-C bonds and this is the reason why they are found in the exhaust as smoke (a carbon product).
The ‘BE Fuelsaver®’ splits the C-C bonds in the fuel and the two Carbon atoms can now make connections with the Oxygen molecules surrounding them. These additional bonds with Oxygen lead to a higher percentage of gas and thereby reduce the amount of fuel needed to move the pistons.